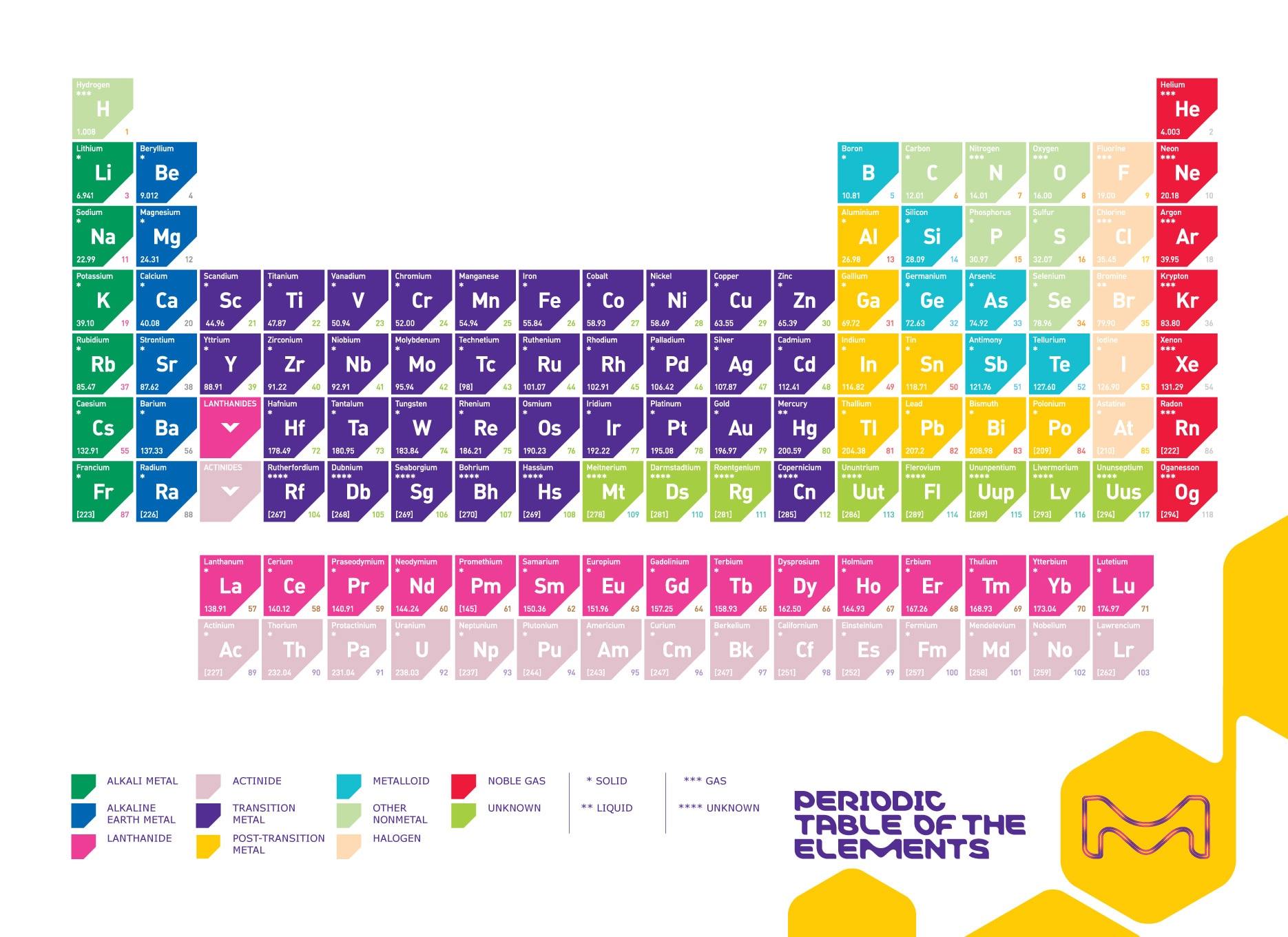
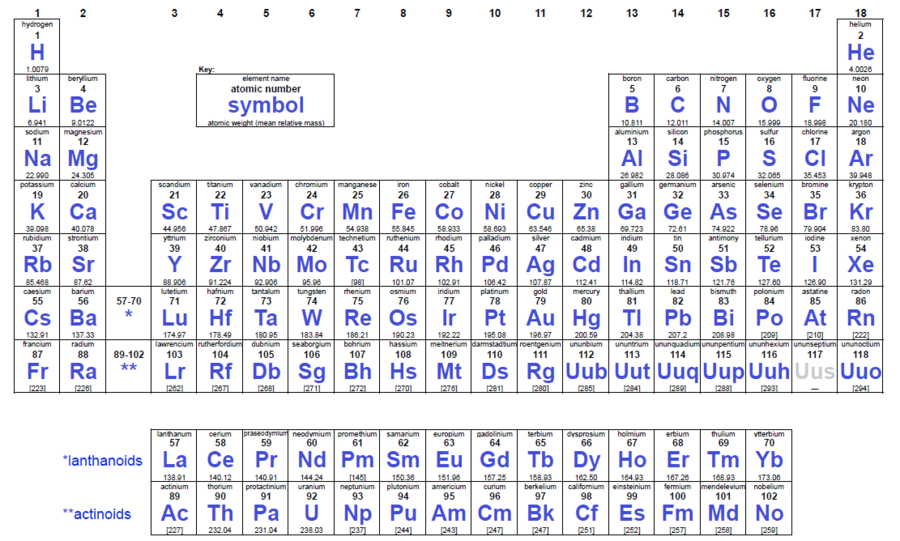
What Is The Periodic Table Of The Elements
Hey all find the importance of periodic table let us know in the comment session below thanks and regards each 1 teach 1.
What is the periodic table of the elements. The noble gases in column 18 all have eight valence electrons. Now lets take a look at the rows. The elements from atomic numbers 1 to 118 have been discovered or synthesized completing seven complete rows of the periodic table. Periods 2 and 3 contain 8 elements respectively.
Atomic weight name sym. But he has one trait that makes him strange almost like that wacky cousin in every family. Each horizontal row of elements in the periodic table is known as a period. Periods 1 to 3 are short periods while periods 4 to 7 are long periods.
Period 1 contains 2 elements. 95 to 118 elements are synthesized only in laboratories or nuclear reactors. Periodic group electron configuration. The history of the periodic table reflects over two centuries.
There are 7 horizontal rows of elements in the periodic table known as period 1 period 2 until period 7. The periodic table groups elements into columns whose atoms have similar valence electron configurations and therefore similar chemistry. Each row is called a period. Period 6 contains 32 elements.
In the case of the halogens in column 17 each of the elements has seven valence electrons. Click a column title such as name to sort the table by that item. Periods 4 and 5 contain 18 elements respectively. The first 94 elements all occur naturally although some are found only in trace amounts and some in nature only after they were first synthesized.
The standard form of the table consists of a grid with rows called periods and columns called groups. In the nineteenth century when the periodic table was created his presence was predicted by the grand old man called mendeleev who baptized him with the name eka manganese which translates to 1 manganese in sanskrit. Abundance in earth. List of periodic table elements sorted by.
The periodic table is an arrangement of the chemical elements organized on the basis of their atomic numbers electron configurations and recurring chemical propertieselements are presented in order of increasing atomic number. He has a metallic descent surrounded by stable elements on all sides. See notes at the bottom of the table. 0c density gcm 3.
Elements are listed in order of increasing atomic number lined up so elements that exhibit similar properties are arranged in the same row or column as others. The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical properties.